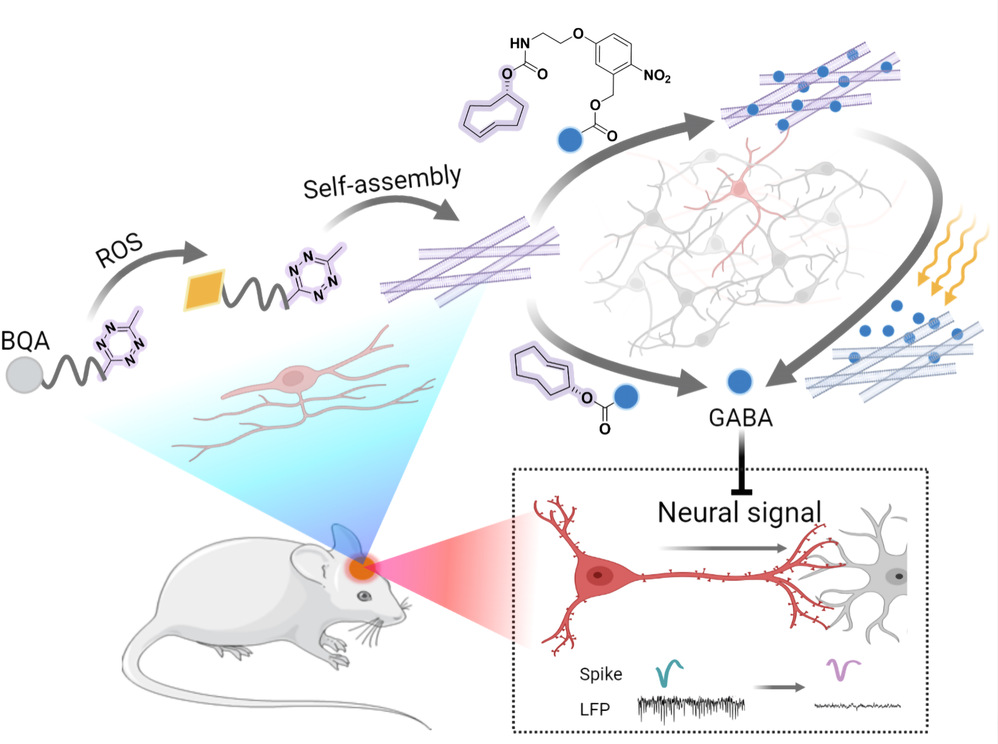
Recently, Professor Yuan Gao from the School of Materials at Beijing University of Chemical Technology published a research paper titled Intrahippocampal Supramolecular Assemblies for Bioorthogonal Liberation of Neurotransmitters to Suppress Seizures in Freely Moving Mice in the internationally renowned journal Advanced Materials. By integrating bioorthogonal reactions with a ligand-free targeting strategy, the research group has developed supramolecular assemblies capable of forming at the epileptic focus. These assemblies facilitate the targeted and controllable release of the inhibitory neurotransmitter GABA through bioorthogonal / photo activation. Additionally, using high spatiotemporal resolution microelectrode arrays at the brain-machine interface, the study has achieved multi-channel, synchronized recording of the long-term and effective mitigation of epilepsy symptoms at the single neuronal level in freely moving mice.
Epilepsy is recognized as one of the most significant challenges in the public health. Despite substantial progress in the pharmacological treatment of epilepsy, most anticonvulsant medications have limited bioavailability and can have considerable side effects, making it difficult to achieve immediate and effective treatment for seizures. To address the shortcomings of targeted drug delivery and immediate treatment in epilepsy, this study designed ROS-responsive peptidic assemblies to target epileptic foci with pathologically high levels of ROS. Synergizing with the bioorthogonal reaction between Tz and TCO, the group further designed bioorthogonal prodrugs of GABA that can be activated bioorthogonally or via photo-activation. The liberation of GABA, which modulates local GABA levels in the brain, provided immediate relief for acute seizure symptoms and extended long-term effect. Additionally, the development of neural microelectrode arrays allows for real-time monitoring of abnormal neuronal firing activity signals, enabling prompt intervention during epileptic episodes. This work, grounded in the concept of in situ supramolecular assembly and leveraging the inflammatory pathological features of epilepsy, achieves targeted localization, dynamic monitoring, and controllable activation of prodrugs. The precise regulation of GABA levels in the hippocampus offers a novel approach to the pharmacological treatment of epilepsy.
Dr. Chengling Wu, assoc. Prof. Qingxin Yao from Prof. Gao 's research group and Dr. Jingyu Xie from Prof. Xinxia Cai's group at the CAS Aerospace Information Research Institute contributed equally. Prof. Gao and Prof. Cai are the co-corresponding authors for this publication. Beijing University of Chemical Technology is the lead institution for this research. The research has been supported by several key funding programs, including the National Key R&D Program, the National Natural Science Foundation of China, and the Fundamental Research Funds for the Central Universities.
Paper link:https://doi.org/10.1002/adma.202314310.


